EXTERNAL LINK
You are about to leave Pajunk.eu. This link is provided strictly for information sharing purposes.
Pajunk GmbH assumes no responsibility for the quality, content, nature, or reliability of any linked site.
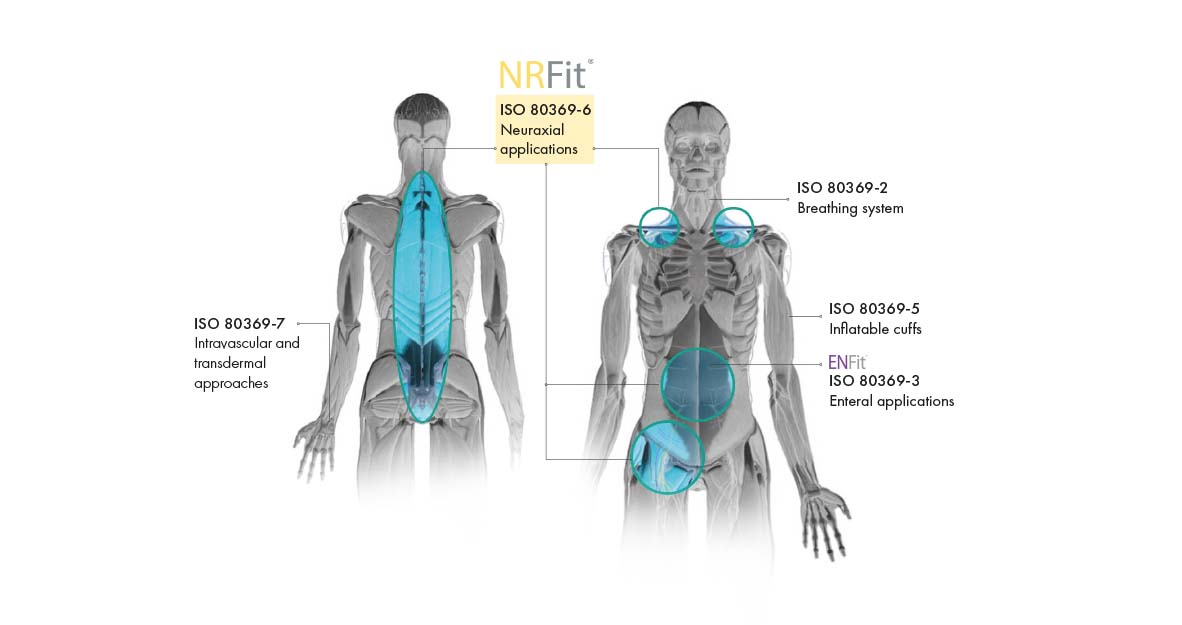
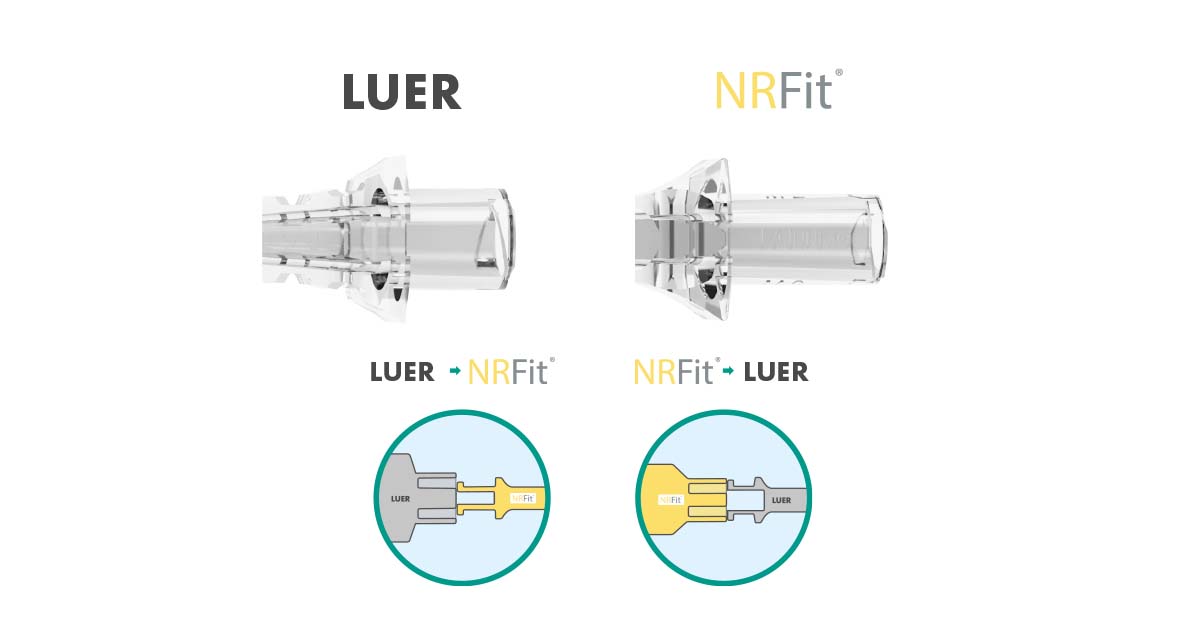
In order to avoid risk of incorrect connections and injections, the DIN ISO 80369 series of standards was issued in 2016. It replaces the overarching LUER standard and defines a separate, incompatible connection for each of the newly defined five application areas. As a leading manufacturer in Regional Anaesthesia, Pajunk is offering its entire product portfolio with the new connection standard. With our year-long experience of more than 500 conversions, you can rely on our expertise. In addition, Pajunk is the only provider to offer complete conversions. We offer a full NRFit product range and support you with different NRFit activities. We are the partner you can rely on.
Pajunk All-Round Service
We can support and assist your successful NRFit conversion.

NRFit symposium at the 4th eESRA
We are proud to say that we could sponsored this symposium with international speaker on their experience pre-, during and post-NRFit conversion. This was the first time that the NRFit topic was presented with a focus on the practical introduction rather than the history and description of the new standard as practiced by all other manufacturers.
NRFit Conversion Video
With the NRFit Conversion Video from our German Sales Team, you can get an overview of the topic. What does the conversion mean? Who is involved? What do I need for the conversion? You will find the answers and more information in the video.
Lessons from a mass neural connector changeover
The first full healthcare facility in New Zealand and Australia that changed to ISO 80369-6 medical device (neural) connector standard was the Auckland District Health Board (ADHB), a facility with more than 1100 beds. This transition was the largest product change in history for them.
In the original article, Dr. Matthew Drake explains what is the key to success and which steps should be considered to run a smooth transition for NRFit.
Read the complete article - start at page 14
Artikel: Australian and New Zealand College of Anaesthesia & Faculty of Pain Medicine (Spring 2021)
| Downloads |
|---|
NRFit Brochure EU
|
NRFit® is a registered trademark of GEDSA and is used with their permission